Review of Ecmo Extra Corporeal Membrane Oxygenation Support in Critically Ill Adult Patients
AbstruseBackground:Administering extracorporeal membrane oxygenation (ECMO) to critically ill patients with acute respiratory distress syndrome has essentially increased over the terminal decade, however administering ECMO to patients with hematologic malignancies may carry a particularly high risk. Here, nosotros study the clinical outcomes of patients with hematologic malignancies and severe astute respiratory failure who were treated with ECMO. Methods:Nosotros performed a retrospective review of the medical records of patients with hematologic malignancies and severe acute respiratory failure who were treated with ECMO at the medical intensive care unit of a tertiary referral hospital between March 2010 and April 2015. Results:A total of 15 patients (9 men; median age 45 years) with hematologic malignancies and severe astute respiratory failure received ECMO therapy during the written report menstruum. The median values of the Acute Physiology and Chronic Health Evaluation II score, Murray Lung Injury Score, and Respiratory Extracorporeal Membrane Oxygenation Survival Prediction Score were 29, 3.3, and -2, respectively. Seven patients received venovenous ECMO, whereas 8 patients received venoarterial ECMO. The median ECMO duration was 2 days. Successful weaning of ECMO was achieved in three patients. Hemorrhage complications developed in 4 patients (1 pulmonary hemorrhage, i intracranial hemorrhage, and 2 cases of gastrointestinal bleeding). The longest menses of patient survival was 59 days afterward ECMO initiation. No significant differences in survival were noted between venovenous and venoarterial ECMO groups (10.0 vs. 10.v days; p = 0.56). Conclusions:Patients with hematologic malignancies and astringent acute respiratory failure demonstrate poor outcomes after ECMO handling. Careful and appropriate selection of candidates for ECMO in these patients is necessary. IntroductionPatients with hematologic malignancies are susceptible to infection because they have an immunocompromised status due to the illness procedure or chemotherapy. In particular, pneumonia and acute respiratory failure (ARF) are the nearly life-threatening complications, and these complications are the primary reason for admitting patients with hematologic malignancies to the intensive intendance unit (ICU).[1,2] Despite notable advances in oxygen therapy such equally loftier-flow nasal cannulas[three] and non-invasive ventilation,[4] most patients with hematologic malignancy and ARF demand invasive mechanical ventilation.[5] The mortality rates of these patients are very high and exceed 50% in such circumstances.[6] Recently, the use of extracorporeal membrane oxygenation (ECMO) as a rescue therapy in ARF patients has received meaning attention.[vii] Since meaningful results were obtained after administering ECMO to pandemic influenza A (H1N1)-induced acute respiratory distress syndrome (ARDS) patients in 2009,[8,9] and reported in a randomized trial,[x] the use of ECMO for the treatment of astringent ARDS has been recommended. [11] Still, administering ECMO to patients with hematologic malignancies and ARF is the subject of debate because they might be expected to demonstrate high bloodshed and a high rate of complications such every bit bleeding and infection.[6,12,xiii] In that location are limited studies on administering ECMO to patients with hematologic malignancies, and these studies study different outcomes.[14-16] It is thus necessary to determine if applying ECMO to such loftier-risk groups is suitable considering the express medical resources and loftier toll of ECMO. The purpose of our study was to nowadays the clinical outcomes of patients with hematologic malignancies and ARF who were treated with ECMO. Materials and MethodsThis study was based on a retrospective review of the clinical courses of all adults patients (≥16 years of age) with hematologic malignancies and ARF who were treated with ECMO at the medical ICU of a tertiary referral hospital (Asan Medical Middle of Seoul, Korea) in Korea between January 2010 and April 2015. All report patients met the criteria of severe ARDS, which were recently divers.[17] ECMO was applied when the patient had astringent and life-threatening refractory hypoxemia despite high levels of positive cease-expiratory pressure or excessively high inspiratory force per unit area, which pb to the failure of proper book- and pressure-limiting strategies during mechanical ventilation.[7] Patients who received ECMO only due to cardiogenic daze were not included in the assay. ECMO support was implemented using the Capiox Emergency Bypass System (Terumo, Tokyo, Japan) and Permanent Life Support (Maquet Cardiopulmonary AG, Hirrilingen, Frg). The vascular admission was established through femoral artery in arterial cannulation, and femoral vein or internal jugular vein in venous cannulation. The size of cannula was adamant by the patient's torso surface surface area and ranged from fourteen F to 25 F in arterial cannula; 17 F to 34 F in venous cannula. Heparin or nafamostat mesylate was given to maintain the desired whole blood activated clotting time of 160 to 180 seconds. We tried to maintain the ECMO blood period to see the goal of arterial oxygen saturation > 85%. Red claret cells and platelets were transfused to maintain the hemoglobin > 7 chiliad/dL, hematocrit > 30%, and platelet > 50 × 103/μL. The patient demographics and characteristics of the hematologic malignancies, including blazon, date of diagnosis, previous therapy, days since therapy and remission status of the malignancy were collected before ECMO initiation. Nosotros calculated the Acute Physiology and Chronic Health Evaluation (APACHE) Ii Score, and Murray Lung Injury Score (LIS) to appraise disease severity at ICU admission.[18,19] In addition, the Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) Score was as well calculated to evaluate the adequacy of ECMO initiation and compare with hospital survival.[20] The etiology of ARF and laboratory tests, including consummate blood cell count, chemistry, coagulation and arterial claret gas parameters (pH, PaO2, PCO2, lactate) were recorded, both before and 24 hours subsequently ECMO were recorded. The parameters associated with ECMO such as manner of ECMO (venoveous [VV], venoarterial [VA] and venovenoarterial), ECMO duration time, use of vasopressor use and anticoagulants, hemofiltration during ECMO, successful weaning of ECMO, and adverse events during ECMO (e.g., bleeding, infection, device-related complications) were besides recorded.[7] Major bleeding was defined every bit a subtract in the hemoglobin levels by > 2.0 g/dL or requiring > 2 units of packed cherry-red blood cells due to an obvious bleeding effect, surgical interventions, or intracranial hemorrhage.[15] Finally, ICU and hospital outcomes were assessed by survival or decease. The data are presented as the median and interquartile range (25-75%) for continuous variables or number and percentage for noncontinuous variables. Survival was analyzed using the Kaplan-Meier method. In this study, p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 19.0 (SPSS Inc., Chicago, IL, U.s.). This written report was approved by the institutional review board of Asan Medical Center (No. 2016-0352). The requirement for informed consent was waived by the institutional review board due to the retrospective nature of the analysis. ResultsDuring the study menstruum, a total of fifteen patients received ECMO treatment. Nine of these patients were men, and the median historic period was 45 years (31-54 years). The individual characteristics of the report population are provided in Table ane. The etiology of ARF was viral or bacterial pneumonia in all patients. All patients also fulfilled the criteria of severe ARDS. 1) Hematologic malignanciesThe types of hematological malignancies in our current written report population included the following: 8 patients had leukemia (acute myeloid leukemia [n = iv], chronic myeloid leukemia [n = 2], biphenotypic astute leukemia [n = i], and the transformation of myelodysplasia syndrome to acute myeloid leukemia [n = one]), 2 patients had lymphoma (precursor T-cell lymphoma [n = one] and diffuse big B-prison cell lymphoma [n = 1]), 1 patient had hemophagocytic lymphohistiocytosis, 1 patient had aplastic anemia, 1 patient had multiple myeloma, and 2 patients had myelodysplastic syndrome. Ten patients had undergone hematopoietic stalk cell transplantation (HSCT). The median number of days since HSCT was 27 (ten.viii-268.5). Among the x HSCT patients, 6 had recently (inside 90 days) undergone this treatment. These cases underwent ARF during admission for HSCT, and ARF occurred subsequently a median of 17 days. The diagnosis of hematologic malignancy was fabricated after ECMO initiation in 1 patient (patient no. 7) with hemophagocytic lymphohistiocytosis. At that place was 1 patient (patient no. 11) who received chemotherapy during ECMO support. No chemotherapy was administered to 1 patient (patient no. 13) who was diagnosed with astute myeloid leukemia beingness transformed from myelodysplasia syndrome because ARF occurred immediately subsequently diagnosis. ii) Baseline characteristicsImmediately earlier ECMO support, the median APACHE II score was 29 (25-39), and LIS was three.iii (3.0-3.5). The median RESP score was -ii (-four-0). Patients were categorized as having an RESP score of grouping 2 (1 patient; 6.7%), Iii (iv patients; 26.7%), 4 (7 patients; 46.7%), or Five (three patients; 20%). Leukopenia was nowadays in ten patients, with a median leukocyte count of 1.1 (0.one-8.ii) × tenthree/μL, and thrombocytopenia was nowadays in all patients with a median platelet count of 51 (23.0-64.0) × 103/μL. The median accented neutrophil count was 0.4 (0.0-7.1) × 103/μL with showing beneath i × x3/μL in 10 patients. Arterial blood gas analysis revealed the following findings prior to ECMO initiation: pH, seven.22 (vii.14-vii.29); pCO2, 52.0 mmHg (44.0-68.four); PaO2, 45.0 mmHg (36.0-57.0); PaOii/FiOii ratio, 47.0 (36.0-57.0); and lactate, 4.7 mmol/L (1.9-vii.0) (Table 2). iii) ECMO handlingECMO treatment was initiated at the decision of the treating intensivist. The elapsing of mechanical ventilation prior to ECMO initiation was < ii days in eleven patients, 2-vii days in 2 patients, and > vii days in 1 patient. There was 1 patient who was placed in the prone position, and no patients received nitric oxide prior to ECMO initiation. All patients received anticoagulation with unfractionated heparin or nafamostat mesylate during ECMO treatment. Vii patients received VV ECMO, whereas eight patients received VA ECMO because of cardiac dysfunction and sepsis-related shock. The median ECMO duration was two (1-11) days, just less than two days in 8 patients. Weaning of ECMO was successful in 3 patients (1 VV and two VA ECMO patients). All patients received vasopressors in order to obtain a proper claret pressure, and viii patients underwent continuous renal replacement therapy during ECMO treatment (Table 2). At 24 hours later on ECMO initiation, arterial claret gas analysis revealed a median pH of 7.35 (7.25-7.xl), 41.5 mmHg pCOii (29.0-fifty.5), 87.0 mmHg PaOtwo (51.viii-94.eight), and 4.ane mmol/L lactate (2.0-12.three) in 10 patients. In that location were no arterial blood gas data at 24 hours afterwards ECMO initiation in 5 patients considering they died within 24 hours. iv) Adverse eventsThere were hemorrhage complications in four patients. Major bleeding occurred in 2 patients. These complications included diffuse alveolar hemorrhage (patient no. 1) and intracranial hemorrhage (patient no. 2). Minor haemorrhage included gastrointestinal hemorrhage (patient nos. 3 and 4). Anticoagulants were temporarily stopped or nafamostat mesylate was used instead of unfractionated heparin when bleeding was astringent. There were 2 mechanical complications: hemolysis (patient no. 10) and lower-limb ischemia (patient no. 14). 5) ICU and infirmary eventOf the three patients with successful ECMO weaning, only 1 patient (patient no. 14) was transferred to the full general ward at x days after ECMO initiation. The median survival menstruation was 10 days (Fig. 1). The longest survival period was 59 days (patient no. three). Seven patients died within two days after ECMO initiation. In that location was no significant difference in survival betwixt the VV and VA ECMO group (10.0 vs. 10.5 days; p = 0.56). Give-and-takeDespite recent improvements in the outcomes of critically ill patients with hematologic malignancies, their survival rates still remain poor.[half dozen] Thus, ECMO could be proposed every bit the ultimate therapy. Previous studies on this outcome were mainly focused on pediatric patients[21-23] considering ECMO is established every bit the standard therapy for neonates and children with ARF that is refractory to conventional management strategies.[24] In this written report, we nowadays the boosted results on adult hematologic malignancy after studies of other investigators.[14-sixteen] Recently, technological advances in membrane oxygenators, pumps and catheters have progressed markedly. Accordingly, many intensivists use ECMO in a safer and simpler manner than in the past in a variety of clinical situations than past. Still, the role and proper apply of ECMO in patients with ARF accept not been definitively established.[7] In add-on, the use of ECMO in patients with hematologic malignancies has potential medical risks likewise as ethical and economic challenges. Although Wohlfarth et al.[15] and Gow et al.[16] reported some viable results in select patients with hematologic malignancies and ARF, Kang et al.[xiv] reported disparate results like our current written report. A elementary comparing of our present findings with those of other studies would not be appropriate since our series, and that of Kang et al.[14] mostly included leukemia patients whereas the report of Wohlfarth et al.[fifteen] mostly comprised lymphoma patients and that of Gow et al.[16] patients with solid tumors. Moreover, the dissimilar outcomes in these reports might not exist due to the lack of experience using ECMO considering our hospital is a tertiary referral infirmary that treats many ECMO patients (> 100 cases/year). In our electric current report series, more than patients who received HSCT (northward = 10) than in other studies, and pneumonia and ARF occurred during cytopenia immediately later high-dose chemotherapy (n = vi). The median accented neutrophil count of the patients who received HSCT within 90 days (6 patients) was 0.0 (range 0-0.5) × ten3/μL. These severe impairment of amnesty would contribute poor control of infection. There was even ane case of graft failure (patient no. five) and a patient whose malignancy relapsed at several months afterward HSCT (patient no. 2). Furthermore, at that place was a patient who received over 7 days of ventilation (patient no. 7) and a patient who did not received chemotherapy for malignancy (patient no. thirteen). Hence, the survival was poor amidst our written report patients. Besides poor conditions of the patients with hematologic malignancies and ARF, it should exist considered that the pharmacokinetics of many of medications administered to patients receiving ECMO were complex.[25] In particular, in that location are very limited information to guide antibiotics dosage and administration interval in patients receiving ECMO. Follow-up report is needed to deal with minimal inhibitory concentration of antibiotics and propose guideline for dose and interval of antibiotics during ECMO. The menstruation of nowadays analysis occurred earlier the publication of the RESP score.[20] ECMO initiation was performed according to clinical indications and the clinical judgment of the intensivist. Looking dorsum at the RESP scores of our electric current study population, these were very poor and the majority of the patients were beneath group Iv. The RESP scores did not correlated with the survival fourth dimension, but virtually of our patients with a RESP score below group IV died within 7 days. The RESP score consists of 12 variables and includes the acute respiratory diagnosis group, immunocompromised condition, and other variables that are adamant before ECMO initiation.[20] Basically, information technology is difficult for patients with hematological malignancy and pneumonia to exceed a RESP score of ane (i.eastward., grouping III) since the presence of immunocompromised condition and viral/bacterial pneumonia result in score -2 and iii, respectively. Therefore, the early awarding of mechanical ventilation (score 3 for < 48 hours, or score one for 48 hours to vii days) and the use of neuromuscular blockade (score 1) before ECMO initiation is needed to increase the RESP score in such patients and, if possible, in immature patients (score 0 for 18-49 years) who are expected to receive ECMO treatment. Bleeding events were observed in iv patients (27%) in our study. Among these cases, major bleeding was noted in ii patients. This value is relatively lower than reported by Wohlfarth et al.[15] Bleeding remains a clinically significant issue despite the evolution in ECMO devices such as hollow-fiber oxygenators, centrifugal pumps and bicaval dual-lumen cannulas over several decades.[vii,24] Although the incidence of complications in our present series, including haemorrhage in patients with hematologic malignancies, was not significantly different from that of immunocompetent patients in the study past Kang et al.[14], it is important to maintain a proper platelet count in patients with hematologic malignancy because bleeding complications upshot in poorer outcomes. Despite the poor outcomes of our written report, applying ECMO to patients with hematologic malignancies could be still the option of rescue therapy when they are relatively immature and have short elapsing of mechanical ventilator before ECMO initiation. Indeed, at least, the weaning of ECMO was successful in three immature patients (patient no 3, 5, and 14) who had complete remission status of hematologic malignancy and a short period of mechanical ventilator to ECMO. The incidence of complications related to ECMO in these patients would be similar to those in immunocompetent patients with ARF.[14] Our report had several limitations of notation. First, this was a retrospective study with a pocket-size number of patients. However, it would be nearly impossible to perform a randomized prospective study since there are considerable medical and ethical issues. Second, all of the patients in our electric current study had poor baseline characteristics including poor hematologic disease status, APACHE II score, LIS, and RESP score, which led to rescue therapy. Tertiary, they were simply a very small portion of patients among critically ill patients with hematologic malignancies who underwent respiratory failure during the study flow. Therefore, the outcomes of our study cannot be generalized since the patients were from a highly select grouping. In determination, patients with hematologic malignancies and severe ARF demonstrate poor outcomes after ECMO treatment. Conscientious and advisable choice of ECMO candidate is needed. The RESP score would help in choosing proper candidate of ECMO so far. Relatively young patients who accept brusque duration of ventilator and received neuromuscular blocker before ECMO initiation wound benefit from ECMO support. However, in add-on to the RESP score, comprehensive judgement about medical, ethical and economical bug is needed to initiate ECMO in these patients. Further randomized controlled research is likewise warranted to clarify the capability of ECMO in patients with hematologic malignancy and severe ARF. NOTESNo potential conflict of interest relevant to this article was reported. Fig. 1.Kaplan-Meier survival analysis of the written report patients with hematological malignancies and astringent acute respiratory failure. The median survival menses was 10 days after ECMO initiation. ECMO: extracorporeal membrane oxygenation. Table 1.Individual patient characteristics and clinical outcomes
APACHE 2 score: The Astute Physiology and Chronic Health Evaluation Two score; LIS: lung injury score; RESP score: respiratory extracorporeal membrane oxygenation survival prediction score; ECMO: extracorporeal membrane oxygenation; CML: chronic myeloid leukemia; HSCT: hematopoietic stem prison cell transplantation; CR: complete remission; VV: venovenous; DAH: diffuse alveolar hemorrhage; VA: venoarterial; ICH: intracranial hemorrhage; AA: aplastic anemia; MM: multiple myeloma; MDS: myeloid dysplasia syndrome; MOF: multiple organ failure; HLH: hemophagocytic lymphohistiocytosis; AML: acute myeloid leukemia; BAL: biphenotypic acute leukemia. Table 2.Characteristics at before ECMO initiation (n = fifteen)
The data are presented as the median and interquartile range (25–75%) for continuous variables or number and percent for non-continuous variables. ECMO: extracorporeal membrane oxygenation. References1. Azoulay East, Mokart D, Péne F, Lambert J, Kouatchet A, Mayaux J, et al. Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from French republic and Belgium--a groupe de recherche respiratoire en réanimation onco-hématologique study. J Clin Oncol 2013;31:2810-8. 2. Benoit DD, Vandewoude KH, Decruyenaere JM, Hoste EA, Colardyn FA. Outcome and early on prognostic indicators in patients with a hematologic malignancy admitted to the intensive intendance unit of measurement for a life-threatening complexity. Crit Care Med 2003;31:104-12. 3. Lee HY, Rhee CK, Lee JW. Feasibility of high-catamenia nasal cannula oxygen therapy for acute respiratory failure in patients with hematologic malignancies: a retrospective single-center study. J Crit Care 2015;30:773-7. 4. Gristina GR, Antonelli Thou, Conti Grand, Ciarlone A, Rogante S, Rossi C, et al. Noninvasive versus invasive ventilation for acute respiratory failure in patients with hematologic malignancies: a 5-yr multicenter observational survey. Crit Care Med 2011;39:2232-9. 5. Yeo CD, Kim JW, Kim SC, Kim YK, Kim KH, Kim HJ, et al. Prognostic factors in critically ill patients with hematologic malignancies admitted to the intensive care unit. J Crit Care 2012;27:739. e1-6. 6. Taccone FS, Artigas AA, Sprung CL, Moreno R, Sakr Y, Vincent JL. Characteristics and outcomes of cancer patients in European ICUs. Crit Care 2009;xiii:R15. 7. Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. North Engl J Med 2011;365:1905-14. 8. Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators; Davies A, Jones D, Bailey M, Beca J, Bellomo R, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) astute respiratory distress syndrome. JAMA 2009;302:1888-95. 9. Pham T, Combes A, Roze H, Chevret S, Mercat A, Roch A, et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort report and propensity-matched analysis. Am J Respir Crit Care Med 2013;187:276-85. 10. Peek GJ, Mugford G, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic cess of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. eleven. Rush B, Wiskar Thousand, Berger L, Griesdale D. Trends in extracorporeal membrane oxygenation for the treatment of acute respiratory distress syndrome in the United States. J Intensive Care Med 2016;February 17; [Epub]. http://dx.doi.org/ten.1177/0885066616631956. 12. Sun HY, Ko WJ, Tsai PR, Sun CC, Chang YY, Lee CW, et al. Infections occurring during extracorporeal membrane oxygenation use in adult patients. J Thorac Cardiovasc Surg 2010;140:1125. -32. e2. thirteen. Zangrillo A, Landoni One thousand, Biondi-Zoccai One thousand, Greco M, Greco T, Frati Chiliad, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Intendance Resusc 2013;15:172-8. 14. Kang HS, Rhee CK, Lee HY, Kim YK, Kwon SS, Kim SC, et al. Clinical outcomes of extracorporeal membrane oxygenation support in patients with hematologic malignancies. Korean J Intern Med 2015;xxx:478-88. xv. Wohlfarth P, Ullrich R, Staudinger T, Bojic A, Robak O, Hermann A, et al. Extracorporeal membrane oxygenation in adult patients with hematologic malignancies and astringent astute respiratory failure. Crit Care 2014;xviii:R20. 16. Gow KW, Lao OB, Leong T, Fortenberry JD. Extracorporeal life back up for adults with malignancy and respiratory or cardiac failure: the extracorporeal life support experience. Am J Surg 2010;199:669-75. 17. ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Astute respiratory distress syndrome: the Berlin definition. JAMA 2012;307:2526-33. 18. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE Ii: a severity of illness classification system. Crit Care Med 1985;13:818-29. 19. Murray JF, Matthay MA, Luce JM, Film MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988;138:720-3. xx. Schmidt M, Bailey Thou, Sheldrake J, Hodgson C, Aubron C, Rycus PT, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The respiratory extracorporeal membrane oxygenation survival prediction (RESP) score. Am J Respir Crit Intendance Med 2014;189:1374-82. 21. Meister B, Zelger B, Kropshofer M, Klein-Franke A, Crazzolara R, Frühwirth Chiliad, et al. Extracorporeal membrane oxygenation as a rescue therapy for leukaemic children with pulmonary failure. Br J Haematol 2010;148:126-31. 22. Wolfson RK, Kahana MD, Nachman JB, Lantos J. Extracorporeal membrane oxygenation after stem jail cell transplant: clinical determination-making in the absence of evidence. Pediatr Crit Care Med 2005;six:200-3. 23. Lindén V, Karlén J, Olsson M, Palmér K, Ehrén H, Henter JI, et al. Successful extracorporeal membrane oxygenation in four children with malignant illness and severe pneumocystis carinii pneumonia. Med Pediatr Oncol 1999;32:25-31. 24. MacLaren G, Combes A, Bartlett RH. Gimmicky extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med 2012;38:210-20. 25. Shekar K, Fraser JF, Smith MT, Roberts JA. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J Crit Care 2012;27:741. e9-eighteen. |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Source: https://www.accjournal.org/journal/view.php?number=1042
 , You Na Oh, M.D.ii, Sang-Bum Hong, M.D.2, iii
, You Na Oh, M.D.ii, Sang-Bum Hong, M.D.2, iii 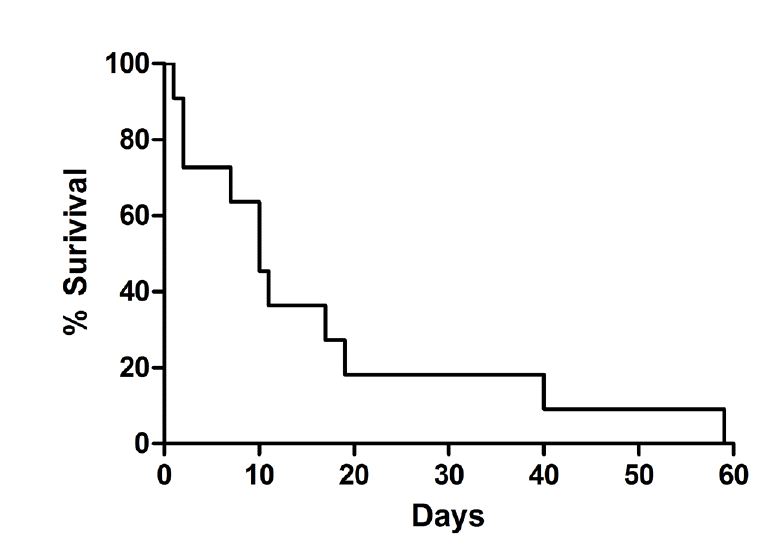


Post a Comment for "Review of Ecmo Extra Corporeal Membrane Oxygenation Support in Critically Ill Adult Patients"